Releases: Open-Systems-Pharmacology/OSP-Qualification-Reports
Version 12.0
Version 11.3.1
This release contains the OSP qualification reports. All reports are created with OSP 11 Update 3 and are available here.
Release Notes
New Qualification Reports
P-gp DDI Qualification Report
This qualification report evaluates the ability of the PBPK platform PK-Sim® (as part of the Open Systems Pharmacology (OSP) Suite) to perform simulations with the intended purpose of predicting permeability glycoprotein (P-gp)-mediated drug-drug interactions (DDI).
Version 11.3
This release contains the OSP qualification reports. All reports are created with OSP 11 Update 3 and are available here.
Version 11.2.1
This release contains the OSP qualification reports. All reports are created with OSP 11 Update 2 and are available here.
Release Notes
New Qualification Reports
No new reports added
Updates
CYP3A4 DDI Qualification Report
Major updates
- Carbamazepine added to the CYP3A4 DDI network: The interactions between carbamazepine as perpetrator and the victim drugs alprazolam, efavirenz, and midazolam are included as well as the interactions between carbamazepine as victim drug and cimetidine, efavirenz, and erythromycin as perpetrators.
Minor updates
See the release notes for CYP3A4 DDI Qualification Plan v. 1.5 for details
Version 11.2
This release contains the OSP qualification reports. All reports are created with OSP 11 Update 2 and are available here.
Version 11.1
This release contains the OSP qualification reports. All reports are created with OSP 11 Update 1 and are available here.
Version 11.0
This release contains the OSP qualification reports. All reports are created with OSP 11 and are available here.
Release Notes
New Qualification Reports
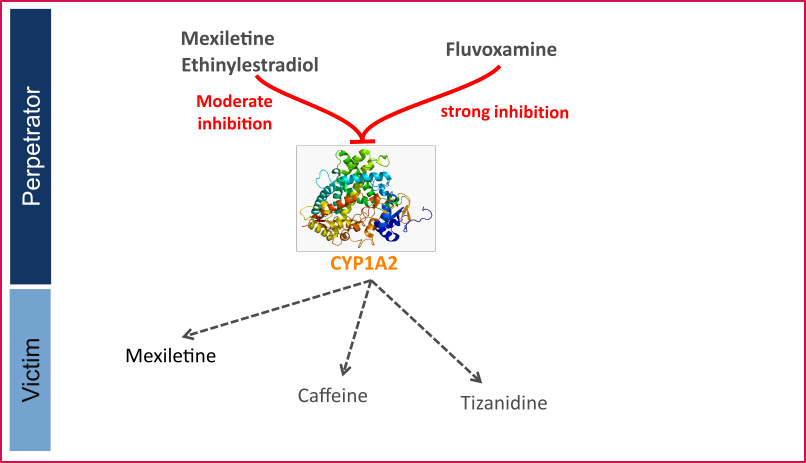
CYP1A2 DDI
This qualification report evaluates the developed PBPK drug-drug interactions (DDI) models network for the ability to perform simulations with the intended purpose to predict cytochrome P450 1A2 (CYPA12)-mediated DDI.
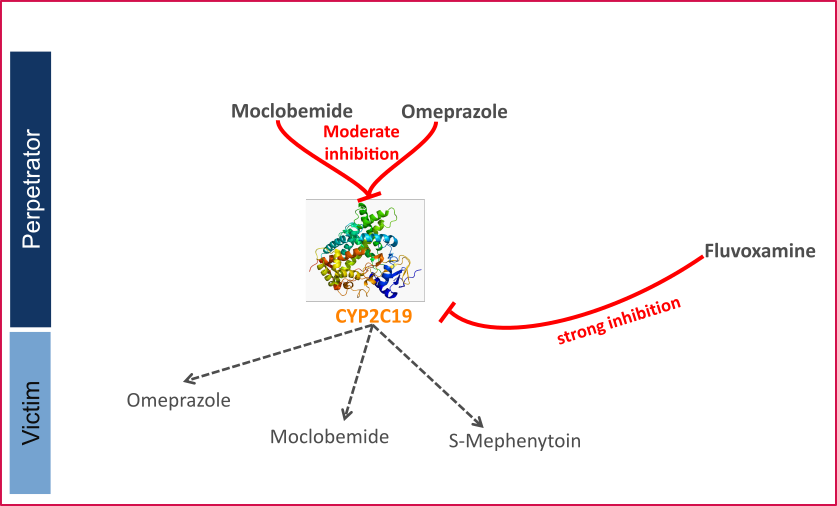
CYP2C19 DDI
This qualification report evaluates the developed PBPK drug-drug interactions (DDI) models network for the ability to perform simulations with the intended purpose to predict cytochrome P450 2C19 (CYP2C19)-mediated DDI.
Updates
UGT DDI Qualification Report
Minor updates
- Fixed wrong chapter content assignment (Open-Systems-Pharmacology/Qualification-DDI-UGT#14)
Pediatric Qualification Report: CYP2C8 Ontogeny
Minor updates
- updated reference to new OSP PK database release (v1.4)
- minor editorial updates and bug fixes (typos, broken links)
Pediatric Qualification Report: CYP3A4 Ontogeny
Minor updates
- updated reference to new OSP PK database release (v1.4)
- minor editorial updates and bug fixes (typos, broken links)
Pediatric Qualification Report: GFR Ontogeny
Minor updates
- updated reference to new OSP PK database release (v1.4)
- minor editorial updates and bug fixes (typos, broken links)
Version 10.0.1
This release contains the OSP qualification reports. All reports are created with OSP 10 and are available here.
Release Notes
CYP3A4 DDI Qualification Report
Major updates
- Fluconazole added to DDI network: Interactions between fluconazole and midazolam, triazolam and alfentanil included.
Version 10.0
This release contains the OSP qualification reports. All reports are created with OSP 10 and are available here.
Release Notes
CYP3A4 DDI Qualification Report
Major updates
- 3 Rifampicin / midazolam DDI studies with in total 8 new data points added: Björkhem-Bergman 2013, Chattopadhyay 2018, Lutz 2018
Minor updates
- updates to latest model snapshot releases due to minor updates in the course of the migration to PK-Sim 10: rifampicin, efavirenz, cimetidine, verapamil, erythromycin, clarithromycin, itraconazole, midazolam, triazolam, alprazolam, alfentanil
- minor editorial updates and bug fixes (s. Qualification Plan Release version 1.2 for details)
UGT DDI Qualification Report
Minor updates
- Fixed broken links
Pediatric Qualification Report: CYP2C8 Ontogeny
Major updates
- Report only contains qualification of CYP2C8 ontogeny, as adult model building and evaluation of the substances used for pediatric qualification are now reported separately.
Minor updates
- removed simulations from model snapshot that are not used for model qualification
- updated reference to new OSP PK database release (v1.3)
- minor editorial updates and bug fixes
Pediatric Qualification Report: CYP3A4 Ontogeny
Major updates
- Report only contains qualification of CYP3A4 ontogeny, as adult model building and evaluation of the substances used for pediatric qualification are now reported separately.
Minor updates
- removed simulations from model snapshot that are not used for model qualification
- updated reference to new OSP PK database release (v1.3)
- minor editorial updates and bug fixes
Pediatric Qualification Report: GFR Ontogeny
Major updates
- Report only contains qualification of GFR ontogeny, as adult model building and evaluation of the substances used for pediatric qualification are now reported separately.
Minor updates
- removed simulations from model snapshot that are not used for model qualification
- updated reference to new OSP PK database release (v1.3)
- minor editorial updates and bug fixes
Version 9.1.1
This release contains the OSP qualification reports.
The qualification reports of this release are created with OSP 9.1 and are available here.
Release Notes
Notes are listed below for new or updated qualification reports. All other qualification reports are unchanged compared to previous release.
CYP3A4 DDI Qualification Report
Major updates
- Cimetidine (v1.0) included with DDIs between cimetidine and alfentanil, alprazolam, midazolam, triazolam and verapamil
- Rifampicin / verapamil DDI added (Barbarash 1988)
Minor updates
- updated references to updated releases of mother repositories for Alfentanil (v2.1), Clarithromycin (v1.1), Erythromycin (v1.1), Fluvoxamine (v1.1), Itraconazole (v1.2), Rifampicin (v1.1) and Verapamil (v1.1)
- updated references to updated released of all former DDI repositories, e.g. Rifampicin-Midazolam-DDI etc. due to optimized simulation time resolutions in snapshots
- updated reference to new OSP PK database release (v1.1)
- minor editorial updates and bug fixes
Pediatric Qualification Report: CYP2C8 Ontogeny
Fixed broken link in the introduction section
Pediatric Qualification Report: CYP3A4 Ontogeny
Fixed broken link in the introduction section
Pediatric Qualification Report: GFR Ontogeny
Fixed broken link in the introduction section